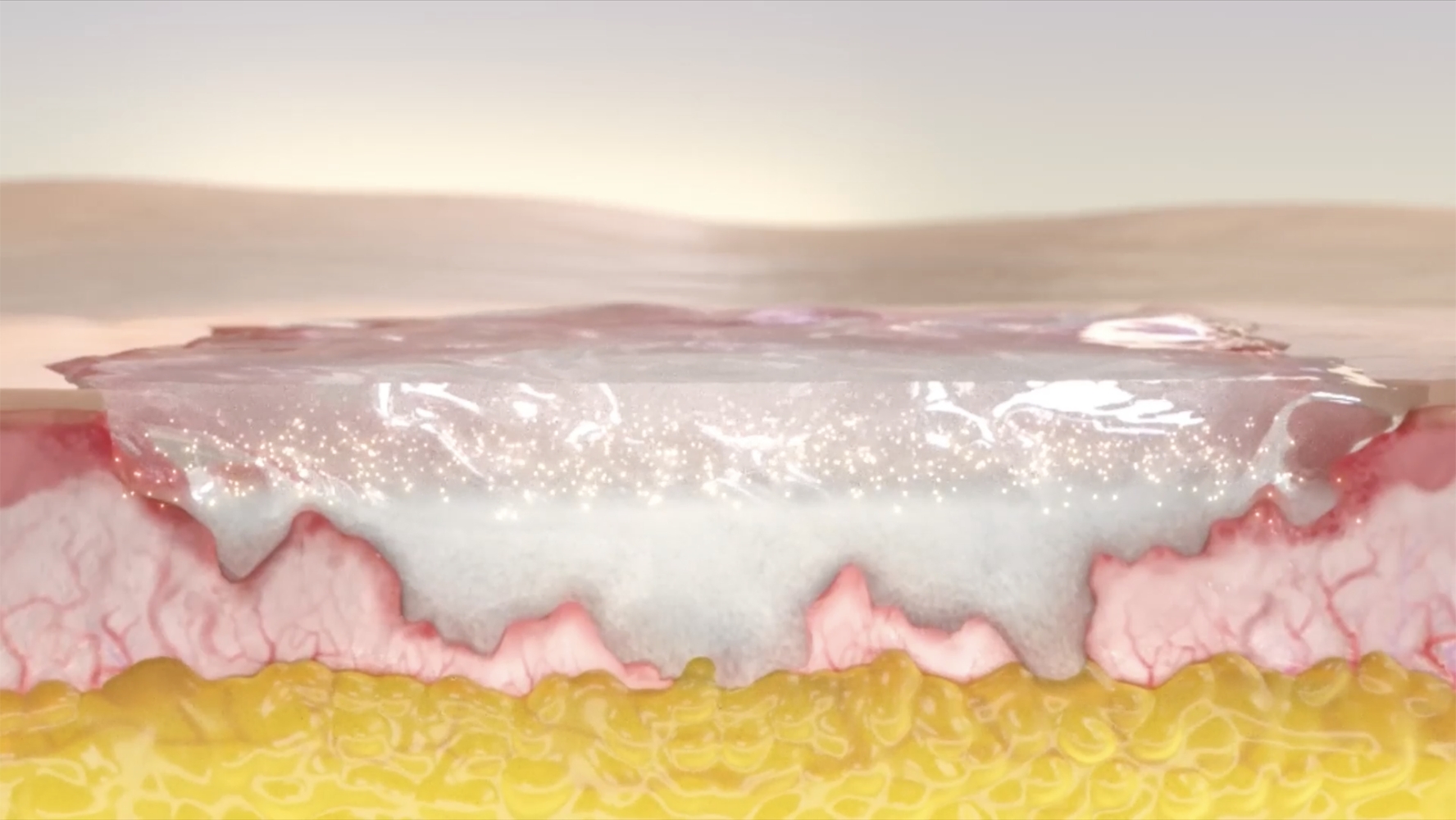
NexoBrid Is a first-line bromelain-based enzymatic agent for eschar removal3,4
Help adult patients with deep partial thickness and/or full thickness burn wounds start towards a path to healing with effective, selective eschar removal1–3
Product illustration

NexoBrid is available as 5 g lyophilized powder (containing 4.85 g of anacaulase-bcdb) mixed in 50 g gel vehicle per 2.5% BSA3
- For topical use only
Studies showed NexoBrid dissolves eschar and can be3:
- Applied as a 4-hour application within hours of patient presentation for earlier and more informed medical planning for healing and care1,3,5
- Remove NexoBrid after 4 hours
- Used at bedside, in the ICU, or in the OR with appropriate monitoring5,6
- Administered by burn surgeons and/or burn care professionals5
Precautions should be taken to avoid exposure during preparation and handling (e.g., gloves, surgical masks, other protective coverings, as needed). In the event of inadvertent skin exposure, rinse NexoBrid off with water to reduce the likelihood of skin sensitization.
This information provided is not intended to provide medical advice or direction. Healthcare professionals should assess each situation and consider their own procedures, including treatment and pain management, for their patients as each patient’s situation will vary.
Please see Full Prescribing Information for guidance on preparation and application.
NexoBrid may provide an alternative option to surgical excision that4
- Removes eschar in adults while preserving viable tissue1–3
- Dissolves eschar within a 4-hour application period3
- Remove NexoBrid after 4 hours
- May lead to more precise wound depth determination1,2
- Achieved ≥95% eschar removal in 93% of patients with DPT and FT thermal burns in a clinical study3
- Lowered the incidence of excision for eschar removal in clinical studies3
Unveiling the NexoBrid wound bed
Selective eschar removal with NexoBrid may reveal a clear view of the extent of the original injury.2,6
Before
After

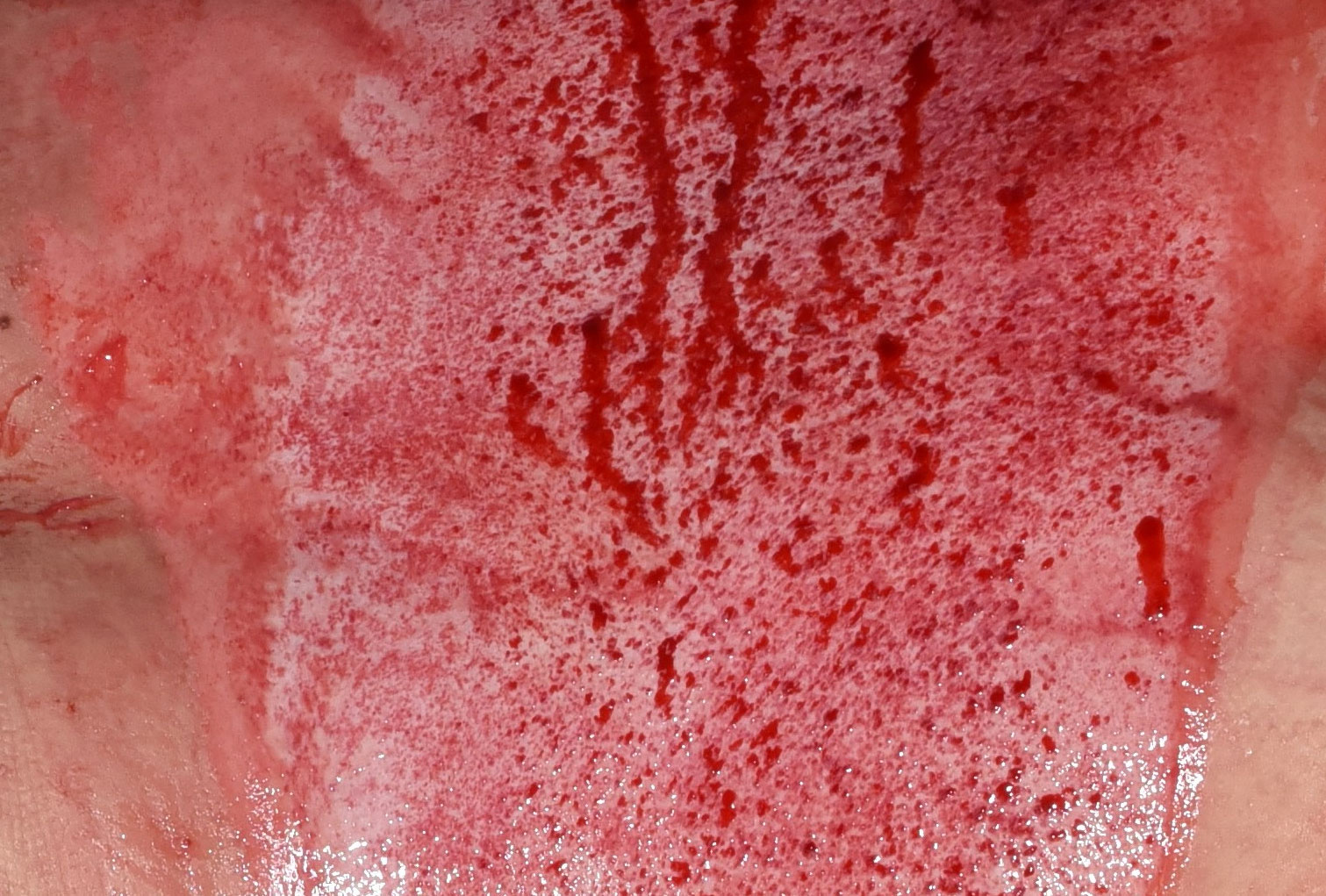
Images are from a real NexoBrid patient

About NexoBrid
NexoBrid is a mixture of proteolytic enzymes extracted from the stems of pineapple plants that dissolves burn wound eschar while preserving viable tissue.1–3

How to use NexoBrid
Find out more about the preparation, application, and removal of NexoBrid.